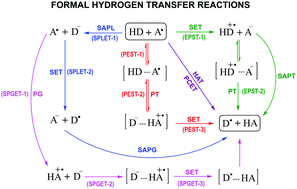
The results presented in this work demonstrate the high complexity of chemical reactions involving species with multiple acid–base equilibria. For the case study investigated here, it was necessary to consider two radical species for tryptophan (Trp(−H)˙ and Trp˙+) and three fractions for uric acid (H3Ur, H2Ur− and HUr2−) in order to properly reproduce the experimental results. At pH = 7.4, two main reaction mechanisms were identified: proton–electron sequential transfer (PEST) and sequential proton gain-electron transfer (SPGET). Combined, they account for more than 99% of the overall reaction, despite the fact that they involve minor species, i.e., H3Ur and Trp˙+, respectively. The excellent agreement between the calculated overall rate constant and the experimental value seems to support this proposal. In addition, if only the dominant species at pH = 7.4 (H2Ur− and Trp(−H)˙) were considered, there would be a large discrepancy with the experimental value (about 4 orders of magnitude), which also supports the finding that the key species in this case are not the most abundant ones. The influence of the pH on the kinetics of the investigated reaction was explored. It was found that the maximum repairing ability of uric acid does not occur at physiological pH, but at a more acidic pH (pH = 5.0).