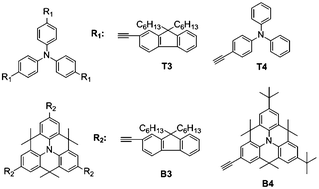
A methylene bridge link connecting the ortho-positions within the triphenylamine scaffold increases the molecular planarity significantly. The one-photon spectroscopies of bridged triphenylamine molecules show considerable extended conjugation relative to their triphenylamine counterparts, leading to enhanced two-photon absorption (TPA) cross-sections. Various substituents at the scaffold have been prepared and studied. Using a femtosecond laser source Z-scan method, the TPA cross-sections were characterized. The maximum magnitude of the TPA cross-section is ∼4800 GM, which is four times that of its triphenylamine counterpart.