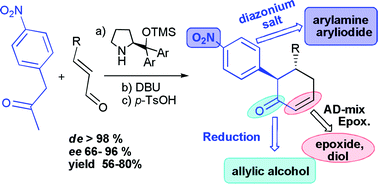
The presence of a p-nitrophenyl group converts acetone into an excellent and versatile nucleophile in organocatalytic processes, able to react with α,β-unsaturated aldehydes affording β-substituted α-arylcyclohexenones via a Michael reaction/aldol reaction/dehydration sequence, which occurs in good yields, ee up to 96% and complete diastereoselectivity. The resulting compounds are excellent synthons for the diastereoselective preparation of a variety of synthetically useful polysubstituted cyclohexanones and derivatives.