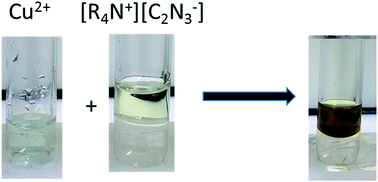
Hydrophobic ionic liquids (ILs) were generated by association between tetraalkylammonium cations and dicyanamide (Dca−) anion. Extraction of Cu(II), Ni(II), Pb(II) and Cd(II) from water was then performed using these ILs at room temperature. The use of a coordinating Dca− anion allows high extraction yields for copper and cadmium to be obtained. The extraction mechanism of Cu(II) has been studied by the determination of the concentration of the organic cation released upon extraction and of the concentrations of counter ions co-extracted. Our results show that the extraction mechanism proceeds via a mixed process involving both cation exchange and ion-pairing, the proportions of which depend upon the nature of the anion and, more precisely, upon its position in the Hofmeister series. The coordination of Cu(II) in ionic liquid phase was followed by UV-vis and EPR spectroscopies.