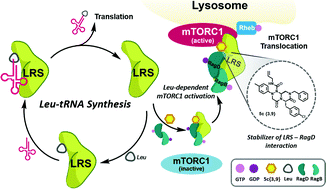
For the systematic perturbation of protein–protein interactions, we designed and synthesized tetra-substituted hexahydro-4H-pyrazino[2,1-c][1,2,4]triazine-4,7(6H)-diones as β-turn mimetics. We then devised a new synthetic route to obtain β-turn mimetic scaffolds via tandem N-acyliminium cyclization and constructed a 162-member library of tetra-substituted pyrazinotriazinediones with an average purity of 90% using a solid-phase parallel synthesis platform. Each library member was subjected to ELISA-based modulator screening for the LRS–RagD interaction, which plays a pivotal role in the nutrient-dependent mTORC1 signalling pathway. Western blot analysis of phosphorylated S6K1 as well as FRET-based imaging confirmed that 5c{3,9} stabilizes the direct interaction between LRS and RagD and activates mTORC1 in live cells under leucine-deprived conditions. Thus, 5c{3,9} can be used as a new research tool for studying the non-canonical role of LRS.