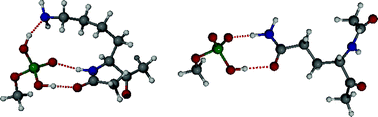
Four synthetic anion transporters (SATs) having the general formula (n-C18H37)2N-COCH2OCH2CO-(Gly)3Pro-Lys(ε-N-R)-(Gly)2-O-n-C7H15 were prepared and studied. The group R was Cbz, H (TFA salt), t-Boc, and dansyl in peptides 1, 2, 3, and 4 respectively. The glutamine analog (GGGPQAG sequence) was also included. A dansyl-substituted fluorescent SAT was used to probe peptide insertion; the dansyl sidechain resides in an environment near the bilayer's midpolar regime. When the lysine sidechain was free or protected amine, little effect was noted on final Cl− transport rate in DOPC : DOPA (7 : 3) liposomes. This stands in contrast to the significant retardation of transport previously observed when a negative glutamate residue was present in the peptide sequence. It was also found that Cl− release from liposomes depended on the phospholipid composition of the vesicles. Chloride transport diminished significantly for the free lysine containing SAT, 2, when the lipid was altered from DOPC : DOPA to pure DOPC. Amide-sidechained SATs 1 and 5 showed a relatively small decrease in Cl− transport. The effect of lipid composition on Cl− transport was explained by differences in electrostatic interaction between amino acid sidechain and lipid headgroup, which was modeled by computation.