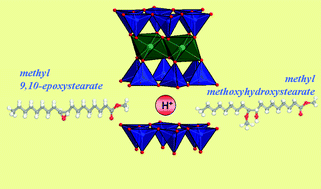
The acid-catalysed reaction of methyl 9,10-epoxystearate ring opening using synthetic saponite acid clay as catalyst, has been studied for the first time. In the presence of methanol, 90% of the epoxide substrate is converted after 5 min and the main reaction product is the vicinal hydroxyether, methyl methoxyhydroxystearate, with 84% of selectivity. In the absence of alcohol the ring opening reaction proceeds slower, leading to a mixture of methyl 9- and 10-oxostearate as main products, and a 9,10-epoxystearate conversion of 66% after 1 h. The performance of acid saponite, an environmentally benign catalyst, is exceedingly higher than those of strong mineral acids, such as H2SO4, widely used for this reaction.