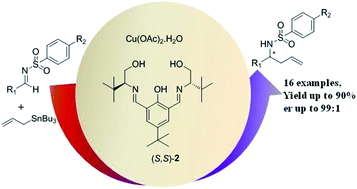
A catalytic route for enantioselective synthesis of homoallyl amines through Cu(II)-Schiff base catalyzed reaction of allyltin with aryl, alkenyl-substituted N-sulfonylimines is described. The allylation reaction is promoted by a simple in situ generated Cu(II)-amino alcohol based Schiff base complex. The addition of allyltin to aldimines delivers the desired products up to 90% yield and 98% enantiomeric excess (ee). Based on experimental observations a probable mechanism was proposed for this reaction. The current methodology was extended to the synthesis of β-phenylalanine in good yield and very good enantioselectivity.