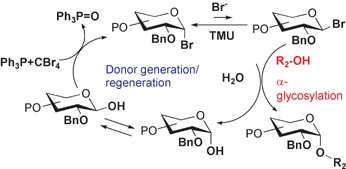
The reaction of a 2-O-benzyl-1-hydroxy sugar with CBr4 and Ph3P generates a glycosyl bromide in situ, which is coupled with an acceptor alcohol in the presence of N,N-tetramethylurea to afford an α-glycosyl product virtually quantitatively. In a proposed pathway, the reagent combination plays multiple roles such as the generation of a glycosyl donor, the activation of glycosylation, and the dehydration of the reaction system. These roles allow a simple α-glycosylation to be performed without special attention to dehydration. Various α-glycosyl (D-gluco-, D-galacto- and L-fuco-) products including glycosyl glycerols and cholesterols have been prepared with this method.