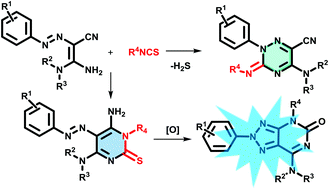
A convenient synthesis of 2-aryl-2,4-dihydro-5H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ones (DTPs) from 3,3-diamino-2-(arylazo)acrylonitriles through a versatile and readily accessible two-step procedure is described. Density functional theory (DFT) calculations were performed to explain the selectivity of the heterocyclization step, which predominantly afforded 6-amino-5-(arylazo)pyrimidin-2(1H)-thiones in chloroform or ethanol, and 2,3-dihydro-1,2,4-triazines in toluene or DMF. Novel 2-aryl-2,4-dihydro-5H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ones were obtained in good yields and showed absorption in the ultraviolet region and good emission in the blue region. The photophysical properties of DTPs were better than those cited in select literature examples of 8-azapurines. Owing to the facile synthesis and good photophysical characteristics in an aqueous medium, the new DTPs should have potential applications as organic fluorophores in fluorescence imaging and materials science.