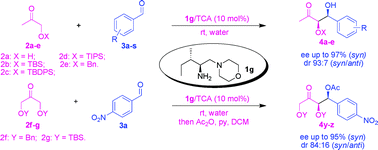
A new series of water compatible primary-tertiary diamine catalysts derived from natural primary amino acids bearing a hydrophobic side chain have been synthesized. These new primary-tertiary diamine-Brønsted acid conjugates bifunctional organocatalysts efficiently catalyzes the asymmetric direct syn selective cross-aldol reaction of different protected hydroxyacetone with various aldehydes in high yield (94%) and high enantioselectivity (up to 97% ee of syn) and dr of 91 : 9 (syn/anti) under mild reaction conditions.