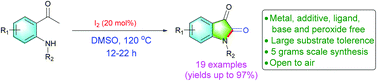
A novel molecular I2-catalyzed synthesis of isatins through C(sp3)–H oxidation and intramolecular C–N bond formation of 2′-aminoacetophenones with excellent yields up to 97% under transition metal, base, additive, peroxide and ligand free conditions is described. The present protocol is suitable for gram scale synthesis of isatins and retained its high yield. Further, the synthetic utility of this present reaction towards synthesis of bioactive 3-hydroxy-2-oxindoles and oxindoles is demonstrated.