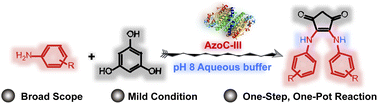
Benzene ring contractions are useful yet rare reactions that offer a convenient synthetic route to various valuable chemicals. However, the traditional methods of benzene contraction rely on noble-metal catalysts under extreme conditions with poor efficiency and uncontrollable selectivity. Mild-condition contractions of the benzene ring are rarely reported. This study presents a one-step, one-pot benzene ring contraction reaction mediated by an engineered nonheme diiron N-oxygenase. Using various aniline substrates as amine sources, the enzyme causes the phloroglucinol-benzene-ring contraction to afford a series of 4-cyclopentene-1,3-dione structures. A reaction detail study reveals that the nonheme diiron N-oxygenase first oxidizes the aromatic amine to a nitroso intermediate, which then attacks the phloroglucinol anion and causes benzene ring contraction. Besides, we have identified two potent antitumor compounds from the ring-contracted products.