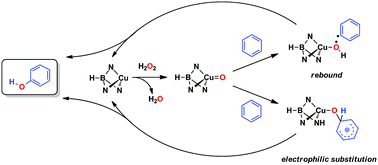
A dual mechanism for direct benzene catalytic hydroxylation is described. Experimental studies and DFT calculations have provided a mechanistic explanation for the acid-free, TpxCu-catalyzed hydroxylation of benzene with hydrogen peroxide (Tpx = hydrotrispyrazolylborate ligand). In contrast with other catalytic systems that promote this transformation through Fenton-like pathways, this system operates through a copper-oxyl intermediate that may interact with the arene ring following two different, competitive routes: (a) electrophilic aromatic substitution, with the copper-oxyl species acting as the formal electrophile, and (b) the so-called rebound mechanism, in which the hydrogen is abstracted by the Cu–O moiety prior to the C–O bond formation. Both pathways contribute to the global transformation albeit to different extents, the electrophilic substitution route seeming to be largely favoured.