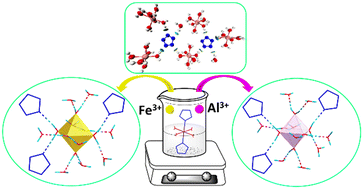
The assembly of cyclo-pentazolate (cyclo-N5−) anions with counterions has thrived in recent years owing to their potential as high energy density materials. However, high-valence metal pentazolate salts are still rare, thus hindering the recognition of the structures and properties of new metal pentazolate salts. Herein, two trivalent metal pentazolate hydrates, namely, Fe(H2O)6(N5)3·9H2O (1) and Al(H2O)6(N5)3·9H2O (2), were synthesized via a metathesis reaction. The compounds were characterized by infrared spectroscopy, single-crystal X-ray diffraction, electrospray ionization mass spectrometry, elemental analysis, and differential scanning calorimetry. The cyclo-N5− anion decomposition temperature for compound 2 is 141.4 °C, which means it has the highest thermal stability among all cyclo-N5−-based materials reported so far. Single-crystal X-ray analysis revealed that there are no direct σ-bonds between the metal centers and cyclo-N5− anions in the crystal lattices of 1 and 2. Hydrogen bonding and π–π stacking interactions in the crystal lattices of 1 and 2 were evaluated. The proportion of hydrogen bonds is more than 75%. Four kinds of hydrogen bond ring motifs are formed via O–H⋯N and O–H⋯O interactions: tetrameric R44(10), pentameric R55(12) and R55(17), and nonameric R99(21) motifs. This study deepens our understanding of the structure of cyclo-N5−-based complexes and is expected to contribute to the further development of pentazole chemistry.