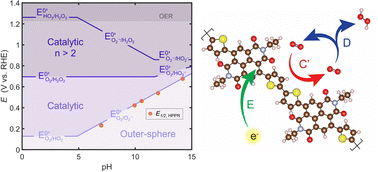
Electrochemical reduction of atmospheric oxygen provides carbon emission-free pathways for the generation of electricity from chemical fuels and for the distributed production of green chemical oxidants like hydrogen peroxide. Recently, organic mixed ionic–electronic conducting polymers (OMIECs) have been reported as a new class of active electrode materials for the oxygen reduction reaction. This work sets out to identify the operative oxygen reduction mechanism of OMIECs through a multi-faceted experimental and theoretical approach. Using a combination of pH-dependent electrochemical characterization, operando UV-Vis and Raman spectroscopy, and ab initio calculations, we find that the n-type OMIEC, p(NDI-T2 P75), displays pH-dependent activity for the selective reduction of oxygen to the 2-electron hydrogen peroxide product. We use microkinetic simulations of the electrochemical behavior to rationalize our experimental observations through a polaron-mediated, non-adsorptive pathway involving chemical reduction of oxygen to the 1-electron superoxide intermediate followed by pH-dependent catalytic disproportionation to hydrogen peroxide. Finally, this pathway is applied to understand the experimental oxygen reduction reactivity across several n- and p-type OMIECs.