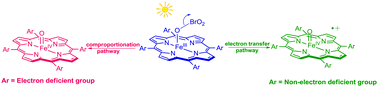
High-valent porphyrin–iron-oxo intermediates are the central oxidizing species in heme-containing enzymes and synthetic oxidation catalysts. In this work, we investigated a new photochemical entry to porphyrin–iron(IV)-oxo derivatives in a variety of porphyrin ligands with different electronic and steric environments. In non-electron-deficient ligand systems, the visible light photolysis of porphyrin–iron(III) bromates gave porphyrin–iron(IV)-oxo radical cations (compound I analogues). In contrast, the photolysis of porphyrin–iron(III) bromates with electron-deficient and sterically encumbered ligands produced one-electron reduced iron(IV)-oxo porphyrins (compound II analogues). Formation of iron(III) μ-oxo dimers was also observed in the sterically unencumbered systems containing electron-deficient substituents. The photochemical generation of porphyrin–iron(IV)-oxo radical cations and iron(IV)-oxo porphyrins permits direct kinetic studies of their oxidations with organic reductants. As expected, more oxidized porphyrin–iron(IV)-oxo radical cations reacted 2–3 orders of magnitudes faster than the iron(IV)-oxo porphyrins, and the rate constants obtained in this work are in comparison to those of iron(IV)-oxo derivatives formed by chemical methods. A model including internal electron-transfer (ET) and comproportionation of the putative iron(V)-oxo species pathways is presented.