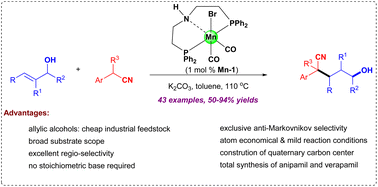
Alcohols and nitrile derivatives are highly important skeletons, widely applied in both organic and bioorganic chemistry. Cross coupling of alcohols and nitriles through formal conjugated addition is a powerful and environmentally friendly strategy for access to long carbon chain tethered nitriles, due to its 100% atom economy and readily available starting materials. Herein, we reported a first example of the pincer manganese(I) catalyzed redox-neutral coupling of nitriles with allylic alcohols to forge a variety of δ-hydroxynitriles. The reaction featured a broad substrate scope with good functional group tolerance under simple conditions (43 examples, 50–94% yields). Remarkably, the mildness and practicality of this protocol were further demonstrated by the successful synthesis of anipamil and verapamil via a one or two-cascade borrowing hydrogen procedure.