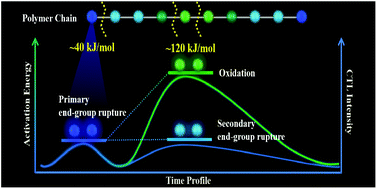
Understanding the mechanism of polymer degradation is vital to the development of rational strategies for polymer management. However, conventional techniques for polymer degradation evaluation rely on the determination of functional group or molecular weight, which fail to provide kinetic information on the steps for polymer degradation. Herein, the degradation evolution and kinetics of polyoxymethylene (POM) are monitored using cataluminescence (CTL) dynamic curves of the generated formaldehyde. The two peaks in the CTL dynamic curves for POM under different atmospheres indicate the occurrence of different reaction pathways, dependent on the reaction kinetics and activation energies of the degradation steps. The activation energies of 31.44 and 48.14 kJ mol−1 were attributed to the end-group rupture, and a higher activation energy of 119.52 kJ mol−1 was responsible for the harsh oxidation reaction of POM. Therefore, we have demonstrated the capability of CTL for the real-time monitoring and effective differentiation of the degradation pathways for polymers. It is anticipated that this method will provide viable possibilities for the design of polymers with high reliability and sustainability.