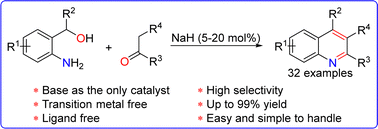
Herein, we report the alkali-catalysed dehydrogenative coupling of amino alcohols and ketones. In this reaction, NaH (5–20 mol%) was employed as the sole catalyst, achieving efficient hydride transfer from the alpha-carbon of alcohol to the carbonyl carbon of ketone, and then the generated aldehyde intermediate was rapidly captured by the ketone to afford the products in good selectivity and yield. This protocol uses readily available starting materials and inexpensive catalysts without any transition metals and ligands and is easy and simple.