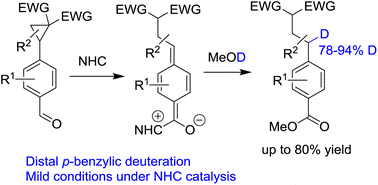
Deuterium incorporation at selective sites of organic compounds has long attracted the interest of the pharmaceutical industry. Here, we present a distal p-benzylic deuteration via N-heterocyclic carbene catalyzed ring-opening of cyclopropylbenzaldehydes with MeOD as the deuterium source. The corresponding 4-alkylbenzoates with high deuterium incorporation at the benzylic position were obtained in good yields. The stable benzylic deuterium remained intact for further chemical transformations.