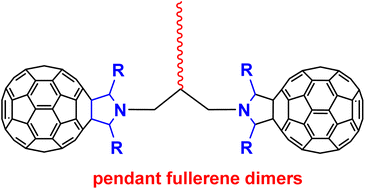
An aminomethylation reaction of fulleropyrrolidines bearing ketone moieties in the presence of N-unsubstituted fulleropyrrolidines and paraformaldehyde with the aid of p-toluenesulfonic acid afforded a series of scarce pendant fullerene dimers. A simple change of reaction substrates from ketone to ketone-containing fulleropyrrolidines successfully realized the synthesis of a variety of novel pendant fullerene dimers, including those from methyl ketone-containing fulleropyrrolidines, which were considered to produce the known bridged fullerene dimers. It should be noted that pendant fullerene dimers are usually difficult to prepare by other methods and may have promising applications in perovskite solar cells. Density functional theory (DFT) has been employed to elucidate the regioselectivity of methyl ketone-containing fulleropyrrolidines to yield exclusively pendant fullerene dimers by investigating the Gibbs free energy profile of the reaction starting from methyl ketone-containing fulleropyrrolidines and iminium intermediates.