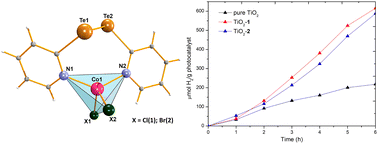
The reactions of bis(2-pyridyl)ditellane (2-PyTe)2 with cobalt salts (CoCl2·6H2O and CoBr2) and CuBr2 resulted in the formation of three new coordination compounds [Co(2-Py2Te2-κN,N′)Cl2] (1), [Co(2-Py2Te2-κN,N′)Br2] (2) and [Cu(2-Py2Te2-κN1,Te2,N2′)Br]2 (3), respectively. In compounds 1 and 2, the ligand is coordinated to cobalt(II) through the nitrogen atoms of the pyridyl groups. On the other hand, in compound 3, the coordination of the soft tellurium donor atom to the soft copper(I) metal center was also observed. Compound [Cu(2-Py2TeClO-κO,N,N′)Cl]2 (4) was formed using bis(2-pyridyl)ditellane as a precursor. After the generation of bis(2-pyridyl)tellane (2-PyTePy-2) in situ, CuCl2·2H2O was added in DMSO. During the reaction, the tellurium atom was oxidized from +2 to +4, with the formation of the anionic ligand [2-Py2Te(Cl)(O)]−. All compounds 1–4 were characterized using spectroscopic techniques and single-crystal X-ray diffraction. Diffuse reflectance spectroscopy data were used to estimate the band gap energies of the compounds. The photocatalysts TiO2-1 and TiO2-2 were prepared and tested for hydrogen evolution from water splitting, using a 300 W Hg/Xe lamp as the irradiation source. Both photocatalysts have produced higher amounts of hydrogen when compared with pure TiO2, suggesting that compounds 1 and 2 perform as effective photosensitizers.