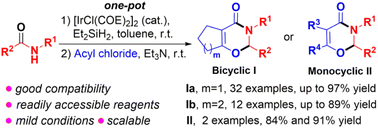
A facile and efficient synthesis of bicyclic 1,3-oxazin-4-ones, 2,3,6,7-tetrahydrocyclopenta[e]-1,3-oxazin-4-ones and 2,3,5,6,7,8-hexahydro-4H-benzo[e]-1,3-oxazin-4-ones, has been achieved via an Ir-catalyzed one-pot reaction of secondary amides with adipoyl chloride and pimeloyl chloride, respectively. This method features good yields, broad substrate scope, mild reaction conditions, and scalability. This reaction also shows good compatibility with acetyl chloride and phenylacetyl chloride to access monocyclic 1,3-oxazin-4-ones.