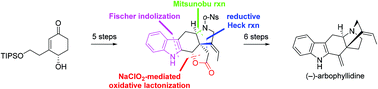
The first asymmetric total synthesis of indole alkaloid arbophyllidine has been accomplished. The synthesis features an intramolecular reductive Heck reaction to install the key quaternary carbon center and a Fischer indolization to efficiently construct the tetracyclic skeleton. Moreover, a novel NaClO2-mediated oxidative lactonization was achieved via selective oxidation at C-16 of the tetracyclic aldehyde. Density functional theory calculations provided an incisive understanding of the oxidative lactonization.