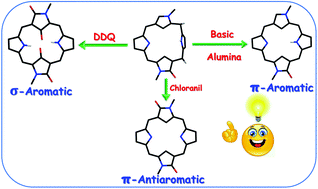
Chemical conversion of non-aromatic trans-doubly N-confused porphodimethenes to hitherto unknown variants of doubly N-confused porphyrinoids/isophlorinoids has been unravelled by the precise interplay between the types of oxidants, the types of meso-aryl substituents and the number of heterocyclic rings in porphodimethene(s). Unique π-reconstructions owing to sequential C-oxygenation of N-confused N-methyl pyrrole rings have led to the genesis of 18π aromatic doubly N-confused monooxo porphyrinoids, 16π antiaromatic doubly N-confused diioxo porphyrinoids and σ-aromatic doubly N-confused tetraoxo isophlorinoids.