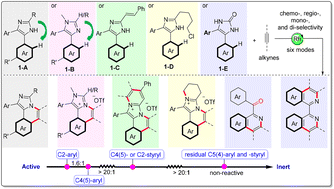
C–H annulations at N- and C2-aryls of an imidazole have been researched well, while the annulation on C4(5)-aryls especially the reactivity and site-selectivity among these aryls remains unknown. Herein, a molecular engineering strategy involving six reaction modes based on the rhodium-catalyzed C4(5)aryl–H activation/annulation of imidazoles with alkynes has been developed, giving diverse neutral/cationic N-heterocycles with broad scope (>60 examples) and high selectivity. More importantly, through a series of intramolecular competition experiments and DFT calculations, the reactivity of the peripheral C–H bonds has been studied and ranked for the first time: C2aryl–H > C4(5)aryl–H > C4(5)styryl–H/C2styryl–H > residual C4(5)aryl–H/C4(5)styryl–H. Furthermore, the remote bulky C2-subsituent is found to have a big influence on the regioselectivity of C4(5)aryl–H activation.