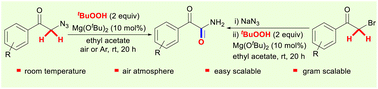
A room temperature Mg(OtBu)2-catalyzed C(sp3)–H oxidation of α-azido arylethanones to straightforwardly produce primary α-ketoamides is presented. By utilizing TBHP as the oxidant and carbonyl oxygen source, this method allows the oxidation of dual methylene C–H bonds α to the carbonyl group for the synthesis of various primary α-ketoamides in moderate to good yields. This method is applicable to one pot oxidative coupling of α-bromoarylethanones with sodium azide leading to primary α-ketoamides.