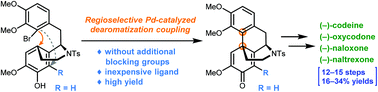
Morphine and its related alkaloids are among the most representative natural medicines that have benefited human beings for over two centuries. Industrial manufacturing of these therapeutically valuable and structurally fascinating molecules relies on farming of ***** poppies, causing severe soil erosion and regulation issues. Despite the advances in the development of numerous biosynthesis and chemical synthesis methods, a truly efficient approach to opioids which is competitive in terms of cost with the current manufacturing protocol remains highly desirable. Here we present a concise total synthesis of opioids exemplified by (−)-codeine, (−)-oxycodone, (−)-naloxone, and (−)-naltrexone with by far the highest overall yields (16–34%) from readily available starting materials. Remarkably, central to the success of the present synthesis is the development of a Pd-catalyzed dearomatization arene coupling reaction using an inexpensive, air stable, and robust phosphonium ligand. This bio-inspired step constructs the tetracyclic morphinan core in a manner with excellent regioselectivity, efficiency, and scalability.