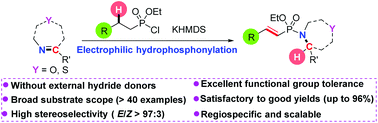
Various α,β-unsaturated (E)-alkenylphosphonamidates were prepared in excellent stereo- and regioselectivities via the electrophilic hydrophosphonylation of cyclic and acyclic aldimines with alkylphosphonochloridates in the presence of base. For unsaturated aldimines, only their respective imine bonds were hydrophosphonylated regiospecifically. The reaction provided a new strategy for the synthesis of (E)-alk-1-enylphosphonamidates via a tandem phosphorylation, deprotonation, and intramolecular 1,5-hydride transfer sequence process from readily available aldimine and alkylphosphonochloridate starting materials. The strategy established the first one-pot electrophilic rather than nucleophilic hydrophosphonylation of aldimines without external hydride donors.