A 512 cage of (H2O)20 consisting of 30 hydrogen bonds encapsulates Astatide with little geometrical distortion. The cage is marginally destabilized but the non-covalent interactions are actually strengthened. Host⋯cage interactions in the [At@(H2O)20]− cluster are anti-electrostatic, placing both negatively charged atoms in direct contact as in Atδ−⋯δ−O–Hδ+. An orbital interaction analysis reveals that explicit host⋯cage contacts are “inverted” hydrogen bonds. That is, the same 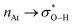 type of donor→acceptor charge transfer as in hydrogen bonding, with no proton bridging the two negative charges.
type of donor→acceptor charge transfer as in hydrogen bonding, with no proton bridging the two negative charges.

