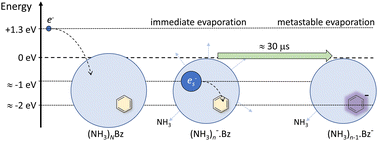
We investigate electron attachment to large ammonia clusters doped with a single benzene (Bz) molecule (NH3)N·Bz, ![[N with combining macron]](http://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 320. Negatively charged clusters are probed by mass spectrometry, and the energy-dependent ion yields are derived from mass spectra measured at different electron energies. The ion efficiency curves of pure ammonia clusters exhibit two maxima. At around 6 eV, (NH3)n−1NH2− ions are produced via dissociative electron attachment (DEA) to NH3 molecules. (NH3)n− ions produced at this energy are formed by DEA followed by fragment caging. At low energies around 1.3 eV, only (NH3)n− ions are formed for cluster sizes n ≥ 35 that correspond to solvated electrons in ammonia clusters. The doped (NH3)n·Bz− cluster ions exhibit essentially the same energy dependence. The (NH3)n·Bz− ions are metastable and evaporate NH3 molecule(s), while pure (NH3)n− ions are stable. The lifetime for NH3 molecule evaporation from the Bz-doped clusters was estimated as τ ≈ 18 μs. We interpret the metastability of the doped clusters by the charge localization on a Bz− ion solvated in the ammonia, which is accompanied by an energy release leading to the evaporation of NH3 molecule(s).
≈ 320. Negatively charged clusters are probed by mass spectrometry, and the energy-dependent ion yields are derived from mass spectra measured at different electron energies. The ion efficiency curves of pure ammonia clusters exhibit two maxima. At around 6 eV, (NH3)n−1NH2− ions are produced via dissociative electron attachment (DEA) to NH3 molecules. (NH3)n− ions produced at this energy are formed by DEA followed by fragment caging. At low energies around 1.3 eV, only (NH3)n− ions are formed for cluster sizes n ≥ 35 that correspond to solvated electrons in ammonia clusters. The doped (NH3)n·Bz− cluster ions exhibit essentially the same energy dependence. The (NH3)n·Bz− ions are metastable and evaporate NH3 molecule(s), while pure (NH3)n− ions are stable. The lifetime for NH3 molecule evaporation from the Bz-doped clusters was estimated as τ ≈ 18 μs. We interpret the metastability of the doped clusters by the charge localization on a Bz− ion solvated in the ammonia, which is accompanied by an energy release leading to the evaporation of NH3 molecule(s).