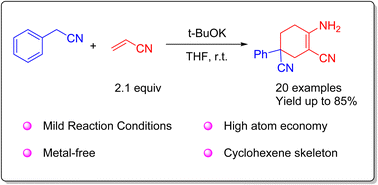
A representative condensation of acrylonitrile and aryl acetonitrile has been reported for the synthesis of α-amino-β-cyano cyclohexene. The reaction was carried out mildly in an open environment at room temperature. The scope and versatility of the method have been demonstrated with 20 examples, containing highly active ethynyl groups. Further applications for 4-aminopyrimidine compounds were performed. A mechanism was proposed, involving Michael additions between acrylonitrile and aryl acetonitriles as well as intramolecular condensation.