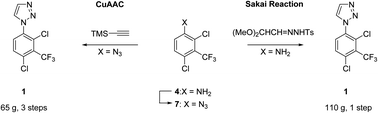
1-Phenyl-1H-1,2,3-triazole 1 (ARUK3001185) was prepared on large scale from aniline 4 by application of both (1) a copper catalyzed azide–alkyne cycloaddition (CuAAC) with (trimethylsilyl)acetylene, and (2) a Clark modification of the Sakai reaction. The one-pot Sakai–Clark method with (MeO)2CHCH![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) NNHTos (2b) proved to be superior as it was operationally simple, metal-free, and avoided the use of aryl azide 7. The Sakai–Clark method has been reliably performed on large scale to produce >100 g of 1 in good efficiency and high purity.
NNHTos (2b) proved to be superior as it was operationally simple, metal-free, and avoided the use of aryl azide 7. The Sakai–Clark method has been reliably performed on large scale to produce >100 g of 1 in good efficiency and high purity.