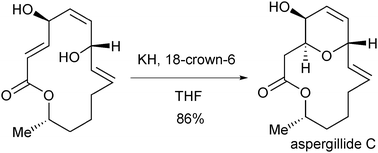
The second-generation enantioselective total synthesis of aspergillide C, a new type of 14-membered macrolide isolated from a marine-derived fungus, has been accomplished in a longest linear sequence of 15 steps from a chiral building block in 19% overall yield employing the 6-exo-trig transannular oxy-Michael reaction as the key step.