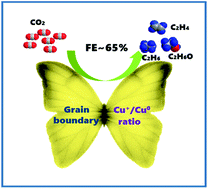
Cu(OH)2/CuO nanocomposite-derived Cu2O/Cu was highly efficient for CO2 electroreduction to C2 products. The highest faradaic efficiency and current density could reach 64.5% and 26.2 mA cm−2, respectively. By changing the calcination time, moderate grain boundary density and the Cu+/Cu0 ratio in catalysts can be controlled, leading to a large number of active sites and low interfacial charge transfer resistance.