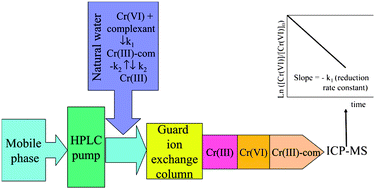
For the first time, a speciation analysis method involving ion exchange chromatography coupled to inductively coupled plasma mass spectrometry was used to perform a kinetic study of Cr(VI) reduction in a certified reference material, SLRS-2, consisting of acidified riverine water. This water sample was spiked with Cr(VI), with or without Cr(III), and evolution of each Cr species as a function of time was monitored by speciation analysis, which showed that the reduction of Cr(VI) was a pseudo first order reaction. By plotting the logarithm of the peak area ratio of the instantaneous Cr(VI) concentration over that of the original spiked concentration versus time, the reaction rate constant was obtained from the slope, and was (2.119 ± 0.040) × 10−4 s−1 in SLRS-2 at pH 1.3 and 20 °C following the addition of 20 μg L−1 Cr(VI). The reduction rate increased with decreasing pH and increasing temperature. The activation energy of the reaction at pH 1.3 was found to be 139.1 ± 7.0 kJ mol−1 using an Arrhenius plot. This method offers the advantages of small sample consumption, minimal sample manipulation, and easy data interpretation.