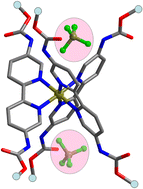
Three 5,5′-dicarbamate-2,2′-bipyridine ligands (L = L1–L3) bearing ethyl, isopropyl or tert-butyl terminals, respectively, on the carbamate substituents were synthesized. Reaction of the ligands L with the transition metal ions M = Fe2+, Cu2+, Zn2+ or Ru2+ gave the complexes MLnX2·xG (1–12, n = 1–3; X = Cl, NO3, ClO4, BF4, PF6, ½SO4; G = Et2O, DMSO, CH3OH, H2O), of which [Fe(L2)3 ⊃ SO4]·8.5H2O (2), [Fe(L1)3 ⊃ (BF4)2]·2CH3OH (7), [Fe(L2)3 ⊃ (Et2O)2](BF4)2·2CH3OH (8), [ZnCl2(L1)][ZnCl2(L1)(DMSO)]·2DMSO (9), [Zn(L1)3 ⊃ (NO3)2]·2H2O (10), [Zn(L2)3 ⊃ (ClO4)(Et2O)]ClO4·Et2O·2CH3OH·1.5H2O (11), and [Cu(L1)2(DMSO)](ClO4)2·2DMSO (12) were elucidated by single-crystal X-ray crystallography. In the complexes MLnX2·xG the metal ion is coordinated by n = 1, 2 or 3 chelating bipyridine moieties (with other anionic or solvent ligands for n = 1 and 2) depending on the transition metal and reaction conditions. Interestingly, the carbamate functionalities are involved in hydrogen bonding with various guests (anions or solvents), especially in the tris(chelate) complexes which feature the well-organized C3-clefts for effective guest inclusion. Moreover, the anion binding behavior of the pre-organized tris(chelate) complexes was investigated in solution by fluorescence titration using the emissive [RuL3]2+ moiety as a probe. The results show that fluorescent recognition of anion in solution can be achieved by the RuII complexes which exhibit good selectivities for SO42−.