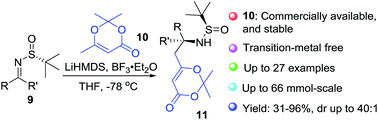
A diastereoselective vinylogous Mannich reaction between the N-tert-butanesulfinyl imines and dioxinone-derived lithium dienolate was developed. A variety of aldimines, ketimines and isatin-derived ketimines are suitable for this process. On the basis of X-ray crystallography of products, a predictive model for this transformation was provided.