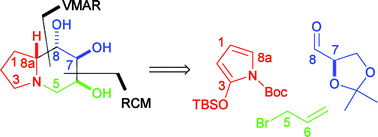
An efficient, stereocontrolled synthesis of (6S,7S,8S,8aR)-6,7,8-trihydroxyindolizidine (alias 1-deoxy-7,8-di-epi-castanospermine) (14) has been developed, which exploits an asymmetric vinylogous Mukaiyama aldol reaction (VMAR) between N-(tert-butoxycarbonyl)-2-(tert-butyldimethylsilyloxy)pyrrole (1) and 2,3-O-isopropylidene-D-glyceraldehyde (2) to construct the initial pyrrolidine building block 3, and an ene-ene ring closing metathesis reaction (RCM) (9 to 10) to install the indolizidine skeleton. The synthetic sequence was 13 steps, proceeding in 19.5% overall yield. The configurational and conformational structure of 14 was ascertained unambiguously and confronted to previously published assignments of rac-14 and ent-14.