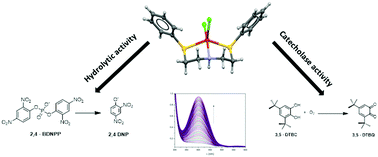
Two new copper(II) mononuclear complexes (CSe and CS) were synthesized and characterized by the following techniques: X-ray crystallography, elemental analysis, IR, EPR and UV-vis spectroscopies, conductimetric analysis and mass spectrometry. The crystalline structures of both complexes revealed two neutral complexes pentacoordinated with their chalcogenated ligands coordinating to the copper(II) center in a tridentate way with Se, N, Se and S, N, S as donor atoms and two cis-chloro ions completing the coordination structures. The hydrolytic activity in phosphate diester cleavage of CSe and CS was investigated using 2,4-BDNPP as a substrate, and applying the Michealis–Menten approach, giving the kinetics parameters (CSe/CS): kcat (s−1) = 1.17 × 10−4/1.97 × 10−4; KM (mol L−1) = 3.13 × 10−3/5.58 × 10−3. Both complexes also catalyze the oxidation of the 3,5-di-tert-butylcatechol (3,5DTBC) substrate with turnover numbers of 4.32 × 10−2 s−1 for CSe and 4.87 × 10−2 for CS.