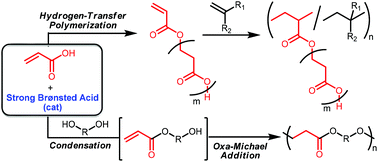
The hydrogen-transfer and condensation–addition polymerizations of acrylic acid (AA) to synthesize aliphatic polyesters have been investigated. We first performed catalyst screening for the hydrogen-transfer polymerization of AA and found that a variety of molecular catalysts, such as phosphines, N-heterocyclic carbenes (NHCs), Lewis pairs, and Brønsted acids, produced oligomeric poly(3-hydroxypropionate). Phosphines and NHCs act as Lewis bases to undergo Michael addition to AA followed by proton transfer to facilitate the polymerization, while strong Brønsted acid catalysts simply activate the carbonyl group of AA to produce the highest molecular weight polyester (Mn = 510, Mw/Mn = 1.4) with a vinyl group at the chain end. The resulting polyester readily undergoes depolymerization in the presence of strong Brønsted acid catalysts. The polyesters can be used as a macromonomer for the radical copolymerization with methyl (meth)acrylates and styrene to produce graft copolymers having the polyester in the side chain. The glass transition temperature of the graft copolymers decreased with the increase of the composition ratio of the polyester. Strong Brønsted acids also catalyzed new condensation–addition polymerization of AA with diols, ethylene and propylene glycols, to produce poly(ester-ether)s. This polymerization is an efficient tandem catalysis and proceeds through the condensation of the carboxylic acid with diols followed by the oxa-Michael addition.