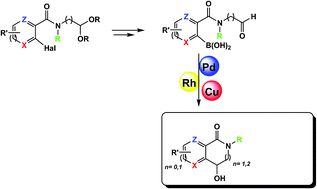
We report an innovative and simple three step high yielding synthesis of a library of 14 chiral isoquinolinone and azepinone derivatives with benzyl, pyridyl and thiophene cores starting from amidoarylboronic acid aldehydes. These products have potential for treating neurodegenerative diseases. The key reaction in this synthetic pathway was an efficient metal-catalyzed (with Rh, Cu and Pd catalysts) intramolecular cyclization. A maximum yield of 87% was obtained using a Rh(I) catalyst.