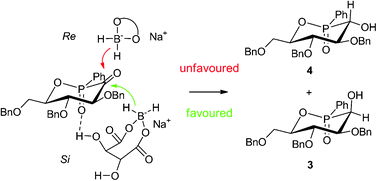
Phostines have been recently described as compounds having antiproliferative properties. Original synthesis of this new class of phosphinic analogs of pyranoses led to a mixture of four diastereomers 3–6 with unequal bioactivities. The most active compound 4 was originally obtained from a mixture of these four diastereomers by selective precipitation, giving firstly two diastereomers 3 and 4, epimers at the carbon atom. From the latter mixture 3 and 4, oxidation with Dess–Martin reagent afforded corresponding α-ketophosphinate 7, which by diastereoselective reduction using a chiral agent based on sodium borohydride and L-proline, gave preferentially the active diastereomer 4. In addition, use of a multivalent cation also increased the diastereoselectivity favourably.