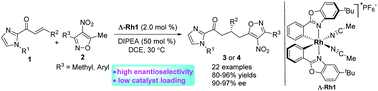
ortho-Alkyl aromatic nitro compounds as vinylogous nucleophiles in catalytic asymmetric synthesis have rarely been reported. Herein an asymmetric vinylogous Michael addition of 5-methyl 4-nitroisoxazoles with α,β-unsaturated 2-acyl imidazoles catalyzed by a chiral-at-metal rhodium(III) complex has been developed. The corresponding adducts were obtained in good yields (80–96%) with excellent enantioselectivities (up to 97%). The reaction can be conducted on a gram scale with a low catalyst loading (0.25 mol%) without impacting its efficiency.