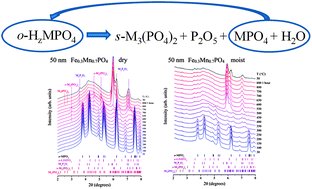
Studies of thermal decomposition mechanism of olivine Fe1−yMnyPO4 are reported here for inert (He), oxidizing (O2) and oxidizing and moist (air) atmospheres using in situ X-ray diffraction and thermal gravimetric analysis with mass spectroscopy. The results indicate that the olivine structure is inherently stable up to at least 400 °C and y = 0.9 for particle size down to 50 nm. However, structural disorder and oxygen loss in the presence of reductive impurities, e.g. carbon and hydrogen, can occur as low as 250 °C for particles larger than 100 nm and at 150 °C for 50 nm particles. Fe1−yMnyPO4 is hygroscopic at high Mn contents, y ≥ 0.6, and moisture exposure is more detrimental to its thermal stability than carbon or small particle size. Nano-Fe1−yMnyPO4 (y > 0.7) with particle size about 50 nm, when exposed to moisture, disorders at 150 °C and transforms to sarcopside phase by 300 °C, no matter whether the delithiation was done electrochemically or chemically. Contrary, under inert atmosphere the sample produced by chemical delithiation is stable up to 400 °C.