A combined first principles and experimental study on Na3V2(PO4)2F3 for rechargeable Na batteries†
R. A. Shakoor,Dong-Hwa Seo,Young-Uk Park,Jongsoon Kim,Sung-Wook Kim,Hyeokjo Gwon,Seongsu Lee,Kisuk Kang
Abstract
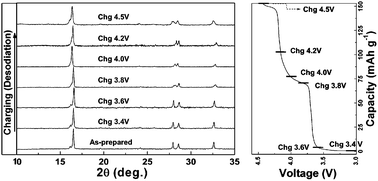
The electrochemical properties of Na3V2(PO4)2F3 in a Na rechargeable battery were investigated through a combined computational and experimental study. Ex situ XRD results indicate that the reversible sodiation/desodiation occurs via one phase reaction and the structure of Na3−xV2(PO4)2F3 remains quite stable upon extraction and insertion of sodium. Notable is that the one phase reaction is accompanied by the negligible variation in lattice parameters (∼1%) and unit cell volume (∼2%) which results in a good cycle performance. It is further noticed that the desodiated phase is thermally stable up to 550 °C implying the excellent safety characteristic of the charged electrode. The first principles calculations elucidate the mechanisms of the structural evolution and the electrochemical behavior of Na3−xV2(PO4)2F3 upon battery cycling.