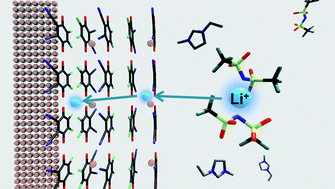
A novel electrochemical interface between metal electrode and room-temperature ionic liquid (RTIL) was prepared by a procedurally facile method. A morphologically uniform and stable metal–quinone charge-transfer complex film of copper-2,3-dicyano-5,6-dichloro-1,4-benzo-quinone (Cu-DDQ) was found to possess lithium active interface against 1-ethyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl] imide ([EMIm][Tf2N]), an RTIL. The cyclic voltammetry of the film showed reversible ionic charge transfers occurring across the interface. The electrochemical impedance spectroscopy indicated that the interfacial impedance may become as low as ∼1000 Ω cm−2. The bias-induced reversible transport of lithium ion was further confirmed by the XPS method. These results suggest that metal–organic CT complex salts prepared on metal electrodes by a simple surface modification may become an excellent electrochemically active interface with RTIL.