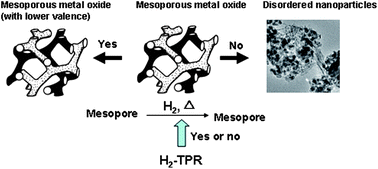
The reduction of a series of crystalline mesoporous metal oxides, CeO2, Co3O4, Cr2O3, CuO, β-MnO2, Mn3O4, and NiO, was investigated using hydrogen temperature-programmed reduction (H2-TPR). CeO2 and Cr2O3 could not be reduced. Mesoporous Mn3O4 and NiO are reduced at almost the same temperature as their bulk counterparts, and mesoporous Co3O4, CuO, and β-MnO2 are reduced at lower temperatures than the corresponding bulk materials. In particular, mesoporous Co3O4 and β-MnO2 undergo reduction to CoO and Mn3O4 while preserving the mesostructure. Further reduction of CoO and Mn3O4, NiO, and CuO does not form mesoporous metal: in the case of CuO, a mesoporous intergrowth of Cu/Cu2O is obtained, and for the rest metal oxides, particles are formed.