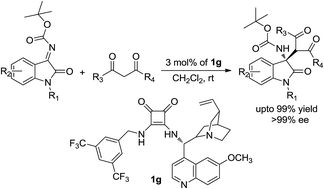
We describe here a simple and facile asymmetric Mannich reaction catalyzed by chiral Cinchona alkaloid based squaramide containing H-bond donor catalysts, wherein, the reaction of 1,3-diketones with isatin (N-Boc) ketimines led to the formation of 3-aminooxindole derivatives. These derivatives were obtained in high yields with excellent enantioselectivities under mild conditions using 3 mol% of the catalyst. This protocol provides valuable and easy access to chiral 3-substituted 3-aminooxindole derivatives.